Global ISO Certification Consultant Services – Qualitcert
-ISO Certification-
ISO 13485 Certification Services in India
QUALIT
CERT
CONSULTING AND ISO CERTIFICATIONS
Strengthen your medical device quality management system with ISO 13485 Certification Services in India by Qualitcert. ISO 13485:2016 is the internationally recognized standard for Quality Management Systems (QMS) specific to the medical device industry. Whether you’re a manufacturer, supplier, distributor, or service provider in the healthcare sector, Qualitcert offers end-to-end consulting services including gap analysis, documentation, employee training, internal audits, and complete certification support.
Our expert consultants ensure compliance with regulatory requirements, risk management practices, and global market expectations. With ISO 13485 certification, you can improve product safety, enhance operational efficiency, and gain access to international markets.
Get In Touch


Please Reach Us Today

Approach and Methodology used to implement Management System Standard
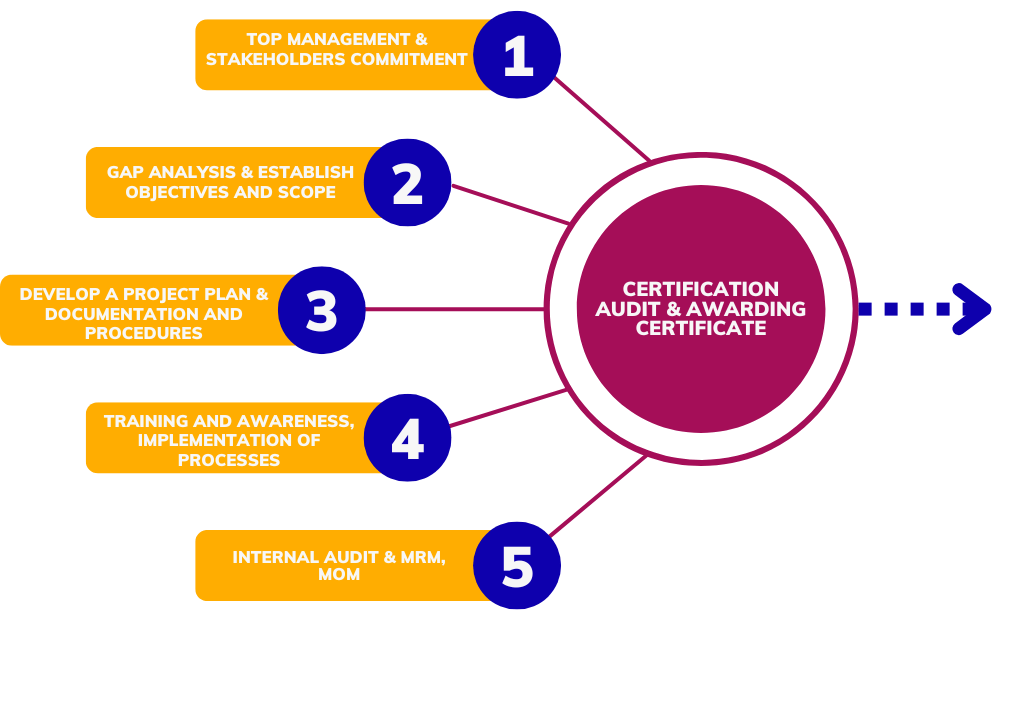
Implementing an ISO standards involves a structured methodology to ensure that the organization effectively meets the requirements of the chosen standard and achieves certification. Sometimes defined methodology may vary depending on factors such as the size of the organization, its industry, and the complexity of the ISO standard being implemented, the following steps provide a basic framework


OUR
Process
1, Determine the ISO Standard
2. Understand the Requirements
3. Training and Awareness
4. Implement the System
5. Internal Audit
6. Certification

Benefits of having ISO Certification
Enhanced Credibility and Reputation
Legal and Regulatory Compliance
Enhanced Customer Satisfaction
Access to Global Markets
Environmental Sustainability
Information Security
Our Achievements and Success
Our Clients





OUR
SERVICES
Our presence in Bangalore
Re-Design your system, create raving fan clients. Transform your business , Go Global
- ISO Certification in Peenya Bangalore
ISO Certification in Bommanahalli Bangalore
- ISO Certification in Whitefield Bangalore
- ISO Certification in Electronics City Bangalore
- ISO Certification in Bommasandra Bangalore
- ISO Certification in Rajajinagar Bangalore
- ISO Certification in Jigani Bangalore
Our presence in Bangalore
Re-Design your system, create raving fan clients. Transform your business , Go Global
- ISO 14001 Certification & Consulting Service in Chennai
- ISO Certification in Oragadam Chennai
ISO Certification in West Chennai
- ISO Certification in North Chennai India
- ISO Certification in Tambaram Chennai
- ISO Certification in Pallavaram Chennai
- ISO Certification in Sholinganallur Chennai India
- ISO 14001 Certification in Adayar Chennai
- ISO 9001 Certification in Velachery Chennai
- ISO Certification in Taramani Chennai

